
Researchers have uncovered a new type of ice that bears an uncanny resemblance to liquid water, potentially rewriting our understanding of water and its many mysteries. Credit: Christoph Salzmann
Researchers at University College London and the University of Cambridge have discovered a new type of ice that more closely resembles liquid water than any other known ices and that may rewrite our understanding of water and its many anomalies.
The newly discovered ice is amorphous — that is, its molecules are in a disorganized form, not neatly ordered as they are in ordinary, crystalline ice. Amorphous ice, although rare on Earth, is the main type of ice found in space. That is because, in the colder environment of space, ice does not have enough thermal energy to form crystals.
For the study, published in the journal Science, the research team used a process called ball milling, vigorously shaking ordinary ice together with steel balls in a jar cooled to -200 degrees Centigrade.
They found that, rather than ending up with small bits of ordinary ice, the process yielded a novel amorphous form of ice that, unlike all other known ices, had the same density as liquid water and whose state resembled water in solid form. They named the new ice medium-density amorphous ice (MDA).
The team suggested that MDA (which looks like a fine white powder) may exist inside ice moons of the outer solar system, as tidal forces from gas giants such as Jupiter and Saturn may exert similar shear forces on ordinary ice as those created by ball milling. In addition, the team found that when MDA was warmed up and recrystallized, it released an extraordinary amount of heat, meaning it could trigger tectonic motions and “icequakes” in the kilometers-thick covering of ice on moons such as Jupiter’s Ganymede (pictured below).

Composite image of Jupiter’s moon Ganymede, taken by the NASA Juno spacecraft on its 34th flyby of the gas giant. Credit: NASA/JPL-Caltech/SwRI/MSSS/Kevin M. Gill
Senior author Professor Christoph Salzmann (UCL Chemistry) said: “Water is the foundation of all life. Our existence depends on it, we launch space missions searching for it, yet from a scientific point of view it is poorly understood.
“We know of 20 crystalline forms of ice, but only two main types of amorphous ice have previously been discovered, known as high-density and low-density amorphous ices. There is a huge density gap between them and the accepted wisdom has been that no ice exists within that density gap. Our study shows that the density of MDA is precisely within this density gap and this finding may have far-reaching consequences for our understanding of liquid water and its many anomalies.”
The density gap between the known amorphous ices has led scientists to suggest water in fact exists as two liquids at very cold temperatures and that theoretically, at a certain temperature, both of these liquids could co-exist, with one type floating above the other, as when mixing oil and water. This hypothesis has been demonstrated in a computer simulation, but not confirmed by experiment. The researchers say that their new study may raise questions about the validity of this idea.
Professor Salzmann said: “Existing models of water should be re-tested. They need to be able to explain the existence of medium-density amorphous ice. This could be the starting point for finally explaining liquid water.”
The researchers proposed that the newly discovered ice may be the true glassy state of liquid water – that is, a precise replica of liquid water in solid form, in the same way that glass in windows is the solid form of liquid silicon dioxide. However, another scenario is that MDA is not glassy at all, but is in a heavily sheared crystalline state.
Co-author Professor Andrea Sella (UCL Chemistry) said: “We have shown it is possible to create what looks like a stop-motion kind of water. This is an unexpected and quite amazing finding.”
Lead author Dr. Alexander Rosu-Finsen, who carried out the experimental work while at UCL Chemistry, said: “We shook the ice like crazy for a long time and destroyed the crystal structure. Rather than ending up with smaller pieces of ice, we realized that we had come up with an entirely new kind of thing, with some remarkable properties.”
By mimicking the ball-milling procedure via repeated random shearing of crystalline ice, the team also created a computational model of MDA. Dr Michael Davies, who carried out the computational modeling whilst a PhD student in the ICE (interfaces, catalytic & environmental) lab at UCL and the University of Cambridge, said: “Our discovery of MDA raises many questions on the nature of liquid water and so understanding MDA’s precise atomic structure is very important.”
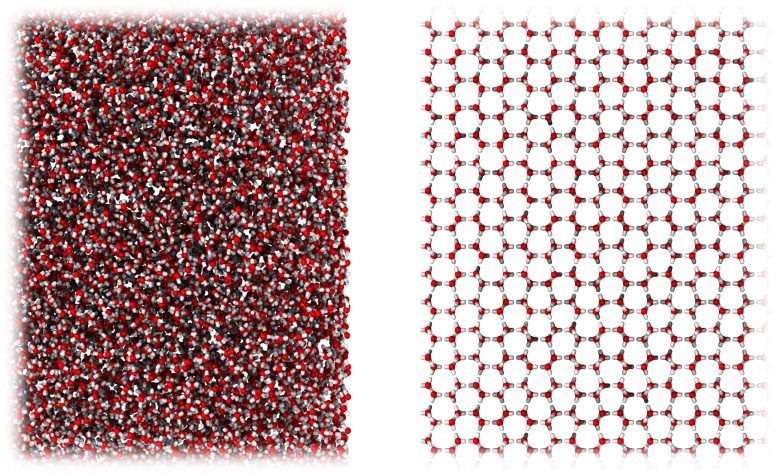
A new form of ice very similar in molecular structure to liquid water (left), compared to ordinary crystalline ice (right). Credit: University of Cambridge
Water has many anomalies that have long baffled scientists. For instance, water is at its most dense at 4 degrees Centigrade and becomes less dense as it freezes (hence why ice floats). Also, the more you squeeze liquid water, the easier it gets to compress, deviating from principles true for most other substances.
Amorphous ice was first discovered in its low-density form in the 1930s when scientists condensed water vapor on a metal surface cooled to -110 degrees Centigrade. Its high-density state was discovered in the 1980s when ordinary ice was compressed at nearly -200 degrees Centigrade. While common in space, on Earth, amorphous ice is thought only to occur in the cold upper reaches of the atmosphere.

Screenshot from video (below) showing the jar with medium-density amorphous ice inside, with steel balls and liquid nitrogen. Credit: Michael Davies
Ball milling is a technique used in several industries to grind or blend materials, but had not before been applied to ice. In the study, liquid nitrogen was used to cool a grinding jar to -200 degrees Centigrade and the density of the ball-milled ice was determined from its buoyancy in liquid nitrogen. The researchers used a number of other techniques to analyze the structure and properties of MDA, including X-ray diffraction (looking at the pattern of X-rays reflected off the ice) and Raman spectroscopy (looking at how the ice scatters light) at UCL Chemistry as well as small-angle diffraction at the UCL Centre for Nature Inspired Engineering to explore its long-range structure.
How to make medium-density amorphous ice (MDA). By repeatedly shearing hexagonal ice we can create MDA.
Furthermore, they used calorimetry to investigate the heat released when the medium-density ice recrystallized at warmer temperatures. They found that, if they compressed the MDA and then warmed it up, it released a surprisingly large amount of energy as it recrystallized showing that H2O can be a high-energy geophysical material that may drive tectonic motions in the ice moons of the solar system.
Reference: “Medium-density amorphous ice” by Alexander Rosu-Finsen, Michael B. Davies, Alfred Amon, Han Wu, Andrea Sella, Angelos Michaelides and Christoph G. Salzmann, 2 February 2023, Science.
DOI: 10.1126/science.abq2105









Fascinating!!!
It’s called a slushie!
Huh. We have that in Northern Vermont.
Except we call it ‘slush’.
How does this form of water differ from that created during the preparation of water samples used in frozen hydrated electron microscopy studies?
As long as water remains wet, I am okay with whatever you want to call it.
The vaccines are dangerous and the masks don’t protect you from anything.
Our governments are all criminals and we are heading into a dark age.
So … what was it you were saying about water?
Amen to that! Scary dark ages ahead!
Shut up.
As long as water remains wet, I am okay with whatever you want to call it.
The vaccines are dangerous and the masks don’t protect you from anything.
Our governments are all criminals and we are heading into a dark age.
So … what was it you were saying about water?
This really reminds me of supercritical CO2, although instead of existing as a state between the liquid and gas states above the triple point, it’s like it sits somewhere between the solid and liquid states, and instead of using high pressure and heat to attain it, low temperatures and mechanical processing are necessary.
I wonder if we’ll reconsider this about ALL of these seemingly simple compounds necessary for life, now? The implications for this could be even bigger if this is applicable in more compounds in a similar fashion.
Potential energy source?!